Controlling biofilm formation using optical traps
As a tool for influencing biofilm growth patterns, laser manipulation could enable biofilms to be used for sensors.
Biofilms – slimy layers formed when bacteria stick together on a surface – allow bacteria to shield themselves from extreme environments and even evade antibiotics. In a new study, researchers showed that laser light in the form of optical traps can be used to control biofilm formation. The findings could allow scientists to harness these microbial layers for various bioengineering applications.
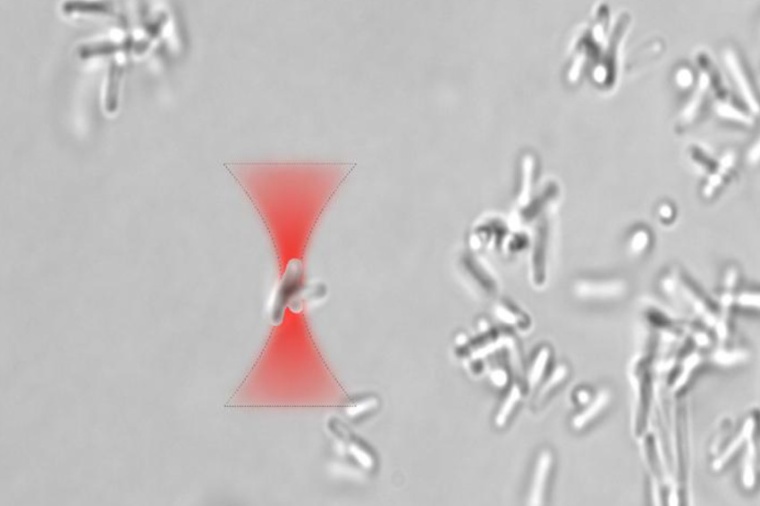
“Producing microscopic components usually requires a highly technical fabrication process, but we found that optical tweezers can be used to precisely control the position of individual bacteria or clusters of bacteria,” said research team leader Anna Bezryadina from California State University Northridge. “This allows us to influence the growth patterns of bacterial structures on a microscopic level with high precision.” Now, the researchers report their experiments with using optical traps to regulate bacterial aggregation and biofilm development. They found that different types of lasers can be used to stimulate and suppress biofilm growth.
“We can even create a sort of bacterial Lego block that can be moved around, stuck together and destroyed as needed,” said Bezryadina. “This work could lead to new types of biodegradable materials or a new generation of biofilm-based biosensors, for example.” Most biofilm research has focused on mechanical, chemical and biological approaches to suppress and control biofilms. Although scientists have shown that synthetic and chemical approaches can be used to activate and control biofilms and engineer biofilms into specific spatial structures, Bezryadina and her team wanted to find out if optical methods could be used to control biofilm dynamics. Accomplishing this required an interdisciplinary team with expertise in advanced optical technology and microbiology.
The researchers experimented with Bacillus subtilis, a non-pathogenic bacterium that naturally forms biofilms. They used a low-nutrient environment that is hostile to B. subtilis to prompt the bacteria to form a biofilm. After obtaining small biofilm clusters, they conducted optical trapping experiments using either a 473 nanometers blue laser or an near infrared Ti:sapphire laser that could be tuned from 700 to 1000 nanometers. They found that using a laser emitting at a wavelength of 820 to 830 nanometers enabled prolonged optical trapping of biofilm clusters while minimizing significant photodamage. However, using a laser at 473 nanometers – a wavelength highly absorbed by the bacteria – caused the cells to rupture and the biofilm clusters to disintegrate. They also observed that the ideal bacterial clusters for optical manipulation consisted of three to 15 cells.
When the researchers studied bacteria dynamics and biofilm formation using optical tweezers at 820 nanometers wavelength for an hour, they discovered that bacterial clusters aggregated near optically trapped clusters, adhered to the surface and started to form a microcolony. They could also move optically trapped bacterial clusters throughout the sample to a specific position, which could be useful for building structures out of bacteria. The NIR laser did not seem to disrupt biofilm formation for bacterial clusters exposed to the highly focused NIR laser, which implies that NIR wavelengths in the 800 to 850 nanometers range could be used for extended periods of time for optical trapping, manipulation and pattern formation of bacterial clusters.
“Despite the apparent uncontrolled bacterial biofilm formation in nature, our work showed that bacterial biofilm formation can be influenced by light,” said Bezryadina. “This paper represents the first step in the long-term project to create microscopic building materials from readily available resources like bacteria. In future studies, we are planning to use what we found to develop a process to construct structures from bacterial Lego blocks.” Overall, the experiments revealed some flexibility in the exact growth conditions, sizes of clusters and wavelengths necessary for manipulating the biofilms. The researchers say that it might also be possible to use their methodology with other types of biofilm-forming microorganisms. (Source: Optica)