FDA clears world’s first photon-counting CT
Siemens Healthineers announces the Food and Drug Administration (FDA) 510 (k) clearance of the Naeotom Alpha, the world’s first photon-counting computer tomograph. With a novel system concept and pioneering new detector technology, it ushers in a new era in computed tomography, a technology that determines the course of many medical decisions.
Thanks to the revolutionary images provided by photon-counting CTs, more people all over the world will benefit from precise and comprehensive examinations at low radiation and contrast dose – from oncological procedures and heart diagnostics to lung follow-up checks for respiratory illnesses.
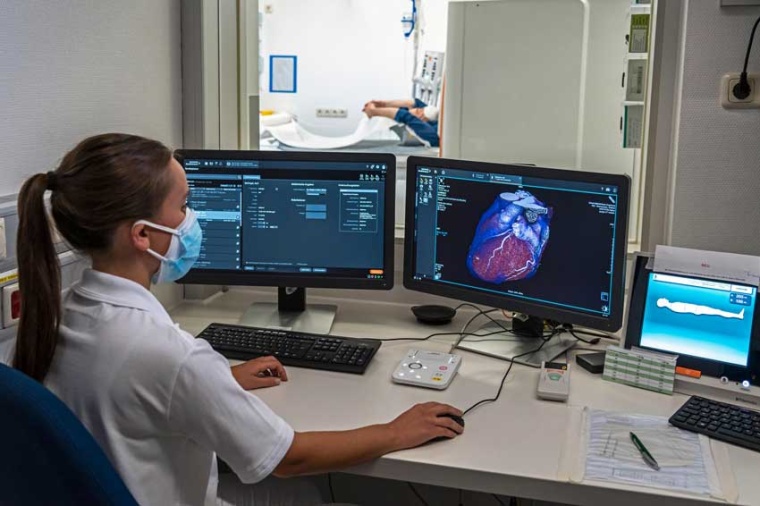
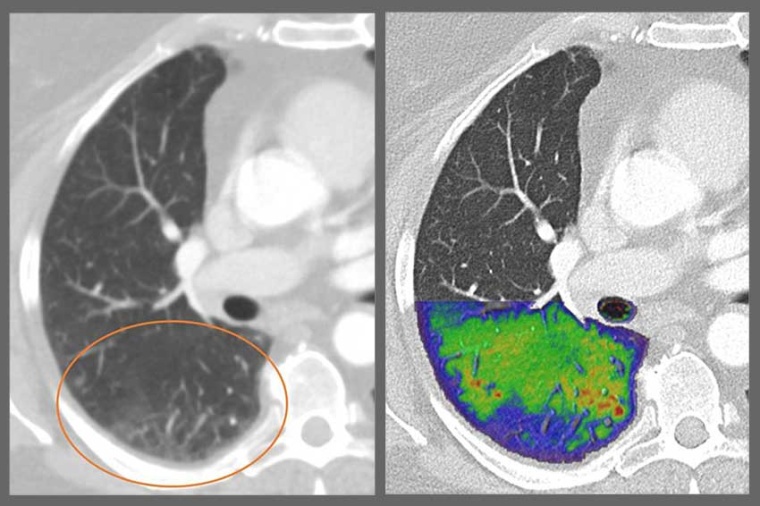


The centerpiece of this innovation is the new photon-counting detector whose active detection layer consists of a cadmium telluride one crystal (CdTe) and offers clear advantages over conventional CT detectors. Standard CT detectors convert the X-rays in a two-step process first into visible light that is subsequently detected by a light sensor, ultimately producing the final image. Due to this intermediate step, important information about the energy of the X-rays is lost and no longer available to aid in diagnosis; contrast is reduced, and images lack clarity.
The photon-counting CT detector developed by Siemens Healthineers no longer converts the X-rays into visible light. The X-ray photons are converted directly into completely digital electrical signals and then counted without information loss. This adds a wealth of completely new clinically relevant information and improves image sharpness and contrast.
“About 15 years ago, work on photon-counting and its clinical vision started at Siemens Healthineers. We always believed in the tremendous clinical value and relentlessly worked on it together with our partners. We are excited that we have received FDA 510(k) clearance,” says André Hartung, Siemens head of diagnostic imaging. (Sources: Siemens / FDA)
Company
Siemens Healthineers GmbHHenkestr. 127
91052 Erlangen
Germany
most read

Ams Osram sells sensor business to Infineon
The sale includes companies with assets of around EUR 130 million, which will be used to repay convertible bonds and senior notes.
Siemens takes over Canopus AI
This expansion of the Siemens EDA software portfolio is designed to help chip manufacturers improve precision and efficiency in wafer and mask inspection processes.

Agile Robots takes over Thyssenkrupp Automation Engineering
This acquisition is intended to strengthen Agile Robots' market position in the field of smart automation solutions

Engineering labor market under pressure: shortage of skilled workers despite the crisis
Unemployment in IT and engineering professions rose by 17.6 percent, while the total number of vacancies fell by 23 percent to 99,470.

5 robotics trends for 2026
The International Federation of Robotics reports on the five most important trends for the robotics industry in 2026.